Despite decades of medical research, clinicians and researchers often struggle to predict who will develop diseases or face complications.
This uncertainty directly affects drug development: clinical trials are often inefficient, lacking reliable biomarkers to identify the right populations and track therapeutic outcomes.
At Pheiron we specialize in phenotypic disease characterization, improving disease understanding and treatment. We develop advanced AI algorithms that integrate multi-omic data and capture disease pathophysiology accurately in AI-driven biomarkers. This shifts the focus from traditional symptomatic endpoints to precision phenotypes based on molecular mechanisms.
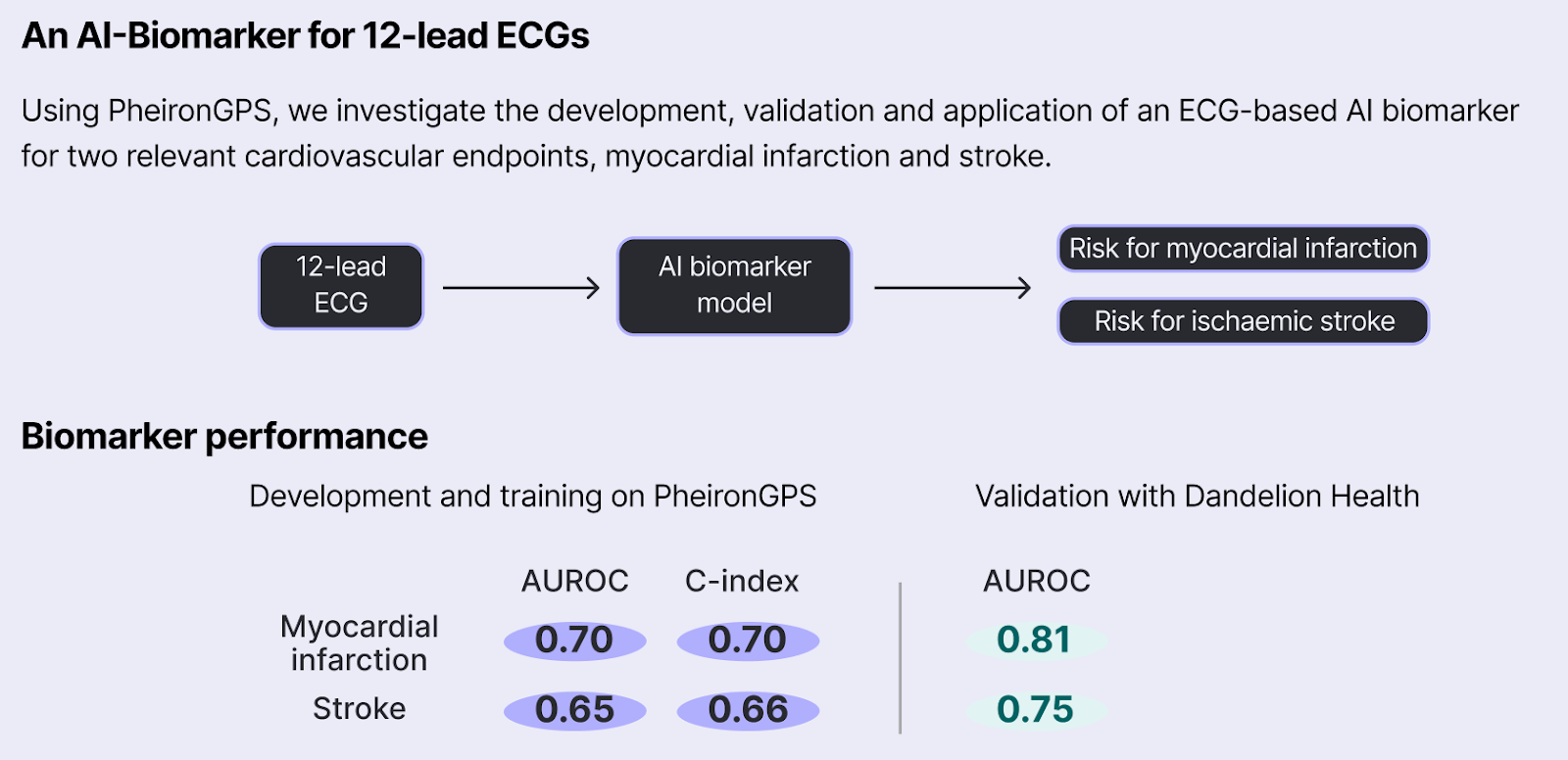
One key application of our AI-biomarkers is in enhancing the evidence generated from RCTs and real-world observational studies. Here, we collaborated with DandelionHealth, a real-world data (RWD) provider, to extract valuable information from multi-omic patient data collected in real-world clinical settings. Specifically, we first validated a Pheiron AI biomarker based on 12-lead ECGs and then conducted a real-world observational study investigating GLP-1’s Cardioprotective Benefits.
We found our AI biomarker to be very robust in Dandelion’s RWD population, with an AUROC of 0.81 for MI and 0.75 for stroke.
Emulating the SELECT trial in Dandelions RWD, we found GLP-1 users to have 15-20% lower MACE risk scores than similar patients not on a GLP-1 after 3-years of GLP-1 use. Importantly, using our AI biomarker we were able to detect statistical significant differences in risk after just 1.7 years, with each year of GLP-1 use associated with a 4% reduction in percentile risk of myocardial infarction and a 3.6% reduction in percentile risk of stroke (p<0.001 for both) on average, as compared to non-GLP-1 patients.
Our study suggests that many more patients could benefit from the cardioprotective effects of GLP-1s than current guidelines indicate. Estimating the potential population-wide benefit of this risk reduction, this means ~44M additional patients may benefit from a GLP-1 in the US alone, which could prevent ~34K strokes and MIs annually!
Our study gives a glimpse at how AI biomarkers will transform clinical research. While retrospective studies like this one can’t replace randomized controlled trials (RCTs), they provide valuable insights to de-risk clinical research: for instance, our results suggest that a GLP-1 receptor agonist trial for primary MACE prevention would likely succeed. Further, the use of AI-driven insights on disease progression allows more targeted patient selection allowing smaller and more efficient trials which can lower costs and speed up the development process by detecting treatment effects sooner. Ultimately, AI biomarkers hold the potential to serve as powerful companion diagnostics transforming how we identify for specific therapies.
We are excited about the future of AI-enabled, accelerated and more cost-effective clinical research!
Reach out, if you would like to learn more or discuss the study.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Suspendisse varius enim in eros elementum tristique. Duis cursus, mi quis viverra ornare, eros dolor interdum nulla, ut commodo diam libero vitae erat. Aenean faucibus nibh et justo cursus id rutrum lorem imperdiet. Nunc ut sem vitae risus tristique posuere.